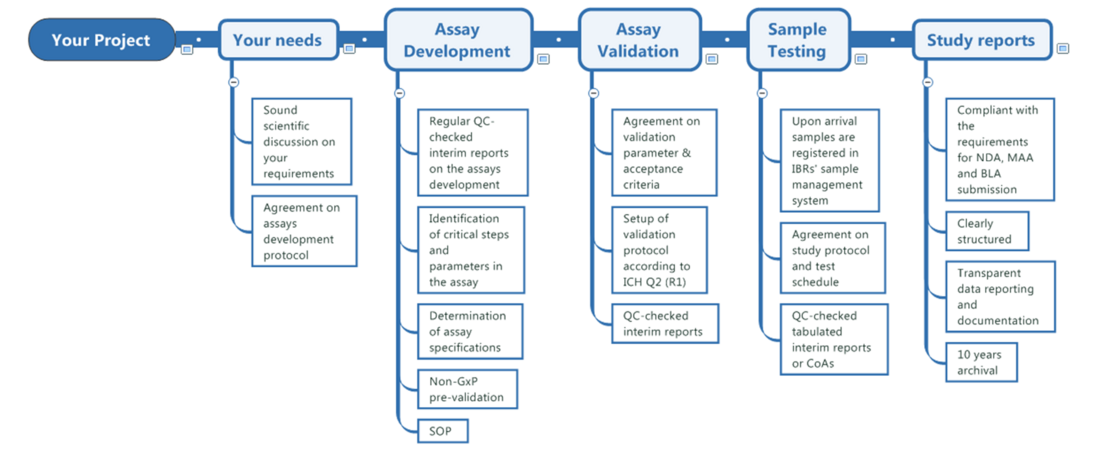
Study Management
As a boutique CRO, IBR Inc. is committed to deliver high quality and reliable results. We set high value on scientific discussion with our customers, to provide them with studies tailored to their needs.
Where applicable, activities are performed in accordance with recognized regulatory and scientific principles, including ICH Q2 (R2), relevant USP chapters (<1032>, <1033>, <1034>), applicable elements of ICH M10 for bioanalytical methods, and GCP principles as defined in ICH E6 (R3). Immunogenicity and anti-drug antibody (ADA) assessments are informed by relevant EMA and FDA guidance, as appropriate.
Submit a Question or Request
Simply fill out the form below and a member of our team will follow up with you shortly.